Edelfosine: A Lipid with Applications in Cancer
The uncontrolled growth of cells in a body is the traditional definition of cancer, and it remains relevant. The difficulty with tackling this disease in a clinical setting is being selective; we want to be able to kill off only the cancerous cells, and not healthy ones. Although there have been many successes in drug–based cancer treatment (cis-platin, taxol, etc), this remains one of the biggest challenges. With this in mind, several approaches are being researched, including semi-physical studies of the surfaces of cancer cells.
Czyz et al. [1] have shown that the introduction of a man-made lipid (known as ‘non-endogenous’) into living systems has several chemical, and presumably physical, effects. One claim they make is that one such non-endogenous lipid, edelfosine (see Figure), accumulates in the cellular and endoplasmic reticulum membranes. This is evidenced by the reorganisation of these parts of the cells. There is the suggestion that this reorganisation also occurs on a much more local scale, with particularly high concentrations of edelfosine in so called ‘lipid rafts’. However, with the theory of lipid rafts still being controversial (at least in living systems) it is unclear what foundation there is for that assertion.
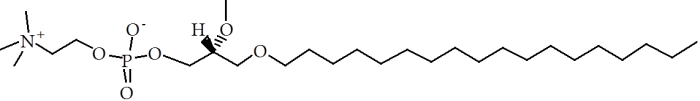
Figure. The molecular structure of edelfosine. Note the head group similarity to phosphatidylcholine and the hydrolyisis-resisting ether-linked fatty alkyl chain.
What is clear, is the chemical influence of this lipid. Through the use of a protein, called pHluorin, and shining light at the cells, the pH of the liquid medium inside the cells (called cytosol) could be determined [2]. The colour and intensity of the light re-emitted by this protein are a function of the concentration of hydrogen ions present. With appropriate calibration, this gives a good insight into the ionic environment inside the cell. What Czyz et al. found was that the intracellular environment becomes much more acidic soon after the lipid had been administered, which immediately begs the question of how this occurs on a molecular level. The suggestion is that the presence of edelfosine has a direct knock-on effect on the cellular machinery that controls the internal environment. This in turn leads to proteins being transported to the wrong parts of the cell, reducing its ability to control pH and prevent the system becoming acidic.
This impressive action of this lipid provides a useful entry into a cancer therapy because it is needed only in small amounts. Edelfosine disrupts an important cellular control mechanism, the falling apart of which leads ultimately to apoptosis.
Although it is not clear what side-effects there may be, or how good a cancer drug therapy this particular lipid will make clinically, the therapeutic approach of using a non-endogenous lipid that accumulates in and interrupts the biochemistry of the target cells, is a tantalizing one that is currently only in the early stages of research.
References
[1] O. Czyz, T. Bitew, A. Cuesta-Marbán, C. R. McMaster, F. Mollinedo, V. Zaremberg. Journal of Biological Chemistry, 2013, DOI: 10.1074/jbc.M112.425744.
[2] R. Orij, J. Postmus, A. Ter Beek, S. Brul, G. J. Smits, Microbiology, 2009, 155, 268-278.