I’ve Forgotten Where I Left that Lipid…
You may have thought that enzymes that hydrolyse (break down) lipids, called lipases, would be a problem for a lot of biological systems. Although there are some obvious exceptions (digestion), this is often true (bacterial infection). However, research published recently suggests that a tightly controlled use of lipases can be very useful for maintaining the membranes and biological machinery that lipids comprise. There is already evidence that lipids that suffer oxidation can be recycled in ocular systems, but there is now evidence that this is type of ‘damage-repair’ occurs in a model gastropod central nervous system.
Watson et al. [1] have recently published evidence that suggests an enzyme called phospholipase A2 has an important influence on long-term memory. This enzyme is capable of hydrolysing one of the two fatty acids from lipids in the membranes of nerve cells that make up the neuronal circuits we need to think. What this means, is that it can remove unsaturated fatty acid residues that have been oxidised. This allows the damaged lipids to be removed such that those required for optimum cellular performance are in place.
One of the earliest references to phospholipase A2 (PLA2) was published in the mid-1950s [2,3], and was in quite a different context. Work by Long and Perry [2,3] used a set of experiments that determined the structure of lecithin that had been exposed to PLA2 isolated from several types of snake venom (cobra, rattlesnake, moccasin). This work indicated that just one of the fatty acid residues was removed, and that it was the fatty acid from one hydroxyl position in particular (red dotted line, Fig. 1), regardless of the type of fatty acid that was present.
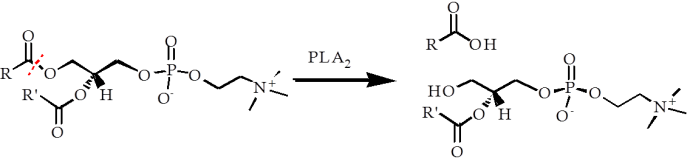
Figure 1. The action of a phospholipase 2 (PLA2) enzyme on a molecule of lecithin (phosphatidylcholine), giving lyso-lecithin and a fatty acid. The fatty acid on the primary hydroxyl is hydrolysed selectively. The R and R’ groups are alkyl chains that form part of the fatty acid residues.
The fact that this enzyme is present in several deadly snake venoms suggests that its activity is damaging, despite the change to the lipid molecule appearing to be partial with respect to the overall structure of the lipid (Fig. 1). It is perhaps not surprising therefore, that the change to the lipid brought about by PLA2 does give rise to a significant change in the physical properties of the lipid. Rather than forming normal, roughly flat lamellar bi-layers, as in a normal cell, this lipid drives an energetic change towards more curved lamellar surfaces, somewhat different to a typical cell. The general effect of this is clear and is observed as the ‘lysis’ (breaking-up) of the cells that the enzyme reaches. This type of enzyme, despite its apparent chemical specificity, can therefore have devastating consequences for the victim of a snake’s bite.
It is therefore something of a surprise that any animal system should evolve an endogenous enzyme for any such purpose. However, the evidence presented by Watson et al. suggests just that: the measured activity of PLA2s, that have specificity for lipids that have suffered damage by oxidation (called peroxidation or peroxidative damage), can be positively healthy. Their results show that systems in which the PLA2s are inhibited contribute to an organism showing signs of memory loss associated with ageing. This strongly indicates that the activity of the enzyme is linked to retaining long-term memory. Importantly, these observations about long-term memory are not consistent with the neuronal cell death that is typically associated with ageing. In the case of PLA2s, it is more a sort of switching-off, rather than an extinguishing of the effect of this enzyme.
So like the digitalis poison from foxgloves and opioids from poppies, it seems that a small, measured amount of yet another deadly poison can have beneficial effects. In the case of PLA2s, a long and healthy life is the reward.
References
[1] S. N. Watson, N. Wright, P. M. Hermann, W. C. Wildering, Neurobiology of Ageing, 2013, 34, 610-613.
[2] C. Long, I. F. Penny, Biochemical Journal, 1954, 58, R15.
[3] C. Long, I. F. Penny, Biochemical Journal, 1957, 65, 382-389